Piglet rearing – there is still room for improvement!

By I. Heinzl, Editor, and Predrag Persak, Regional Technical Manager North Europe
Optimal rearing conditions for piglets are crucial for ensuring their healthy growth, reducing mortality, and enhancing productivity. These conditions include proper temperature, nutrition, housing, hygiene, and care. Here are the key aspects:
1. Temperature and ventilation
Piglets are sensitive to cold because they cannot regulate their body temperature effectively in the first few days after birth. Proper temperature control is essential to prevent chilling, possibly leading to illness and death. Additionally, regulating the temperature would cost energy, which otherwise could be spent for growth.
Signs of a too-cold environmental temperature are piling on top of one another, tucking the legs under the body, being unable to get up, laying near a corner or wall, or shivering, which may stop if the conditions worsen. Measuring the body temperature shows less than 35°C in the case of chilling.
The following temperatures are recommended for successful piglet rearing:
| Farrowing unit (for newborns) | 32 – 35°C (90–95°F) during the first few days |
| After the first week | The temperature can gradually decrease by about 1.5-2.0°C per week until it reaches 25°C (77°F) |
For supplemental heating, heat lamps, heated floors, or creep areas (a designated warm spot) can be used to maintain the ideal temperature, especially in cooler climates.
Temperature is often closely related to ventilation. Ventilation is essential to reduce dust, humidity, ammonia, and other harmful substances occurring in the air. However, if fresh/cold air enters the pigsty, the temperature decreases, which can get dangerous for the piglets. Suitable ventilation means finding a good balance between providing fresh air and maintaining temperature to prevent energy losses and chilling of the piglets.
Comfort zones can be a solution. They are an effective way to keep the piglets warm and ventilation rates where needed to maintain proper air exchange and humidity levels.
2. Nutrition
Nutrition is critical for piglet growth and immune system development. Most important after birth is the access to colostrum. Piglets are born with an immature immune system, and the maternal antibodies ingested with the colostrum are vital for their survival. They should consume colostrum within the first 6 hours after birth.
It will take 5 to 7 days for piglets to stabilize and get regular on suckling schedule.
At around seven days of age, it is recommended to introduce a highly digestible, nutrient-dense creep feed that helps transition piglets from milk to solid food. Fresh and clean water of the best quality must always be available.
Never forget most important nutrient, beside sow´s love and care – water. Allow piglets free access to the excellent quality water.
3. Housing and Space
A well-designed, clean, and dry environment is critical for reducing stress and promoting health. Farrowing crates help prevent sows from accidentally crushing the piglets during the first few weeks. However, these farrowing crates should provide enough space for the sow to nurse the piglets while allowing piglets to move freely.
Separate warm and clean areas (creep spaces) for the piglets within the farrowing pen are helpful to help the piglets escape from cooler or potentially dangerous parts of the crate. Straw, sawdust, or rubber mats should be provided to keep the piglets warm and comfortable, and good drainage is essential to maintain dryness.
4. Hygiene and Health
Hygiene is crucial to prevent disease and promote the health of piglets. For this purpose, pens and farrowing units should be thoroughly cleaned. Regular removal of waste and keeping bedding dry helps control pathogens. It is essential to clean and disinfect the farrowing unit from one farrowing to the other to reduce disease risks.
Health: After birth, the piglets’ umbilical cord stump should be disinfected to prevent infections. A further essential precautionary measure to prevent anemia is an oral supplementation or an iron injection within the first three days of life, as piglets are born with low iron levels.
For further health monitoring and management, it should be ensured that the piglets are vaccinated against common diseases, such as E. coli, Mycoplasma, and Porcine Circovirus. Additionally, deworming protocols and monitoring for signs of parasites should be implemented for parasite control.
5. Weaning Practices
Piglets are typically weaned between 3 and 4 weeks of age, but early weaning (around 21 days) can be practiced in intensive systems. Optimal weaning requires gradual adaptation to solid feed and a stress-free environment.
If the piglets are weaned at 21 to 28 days, a high-quality starter diet after weaning is essential to maintain growth rates and minimize post-weaning stress.
6. Minimizing Stress
Stress management is essential to prevent disease and poor growth. For this purpose, minimize handling to the minimum during the first few days and, if necessary, handle the piglets gently to reduce stress.
A new environment also means strain for the piglets, so keep the litter groups together during weaning to reduce fighting and social stress.
7. Supportive functional feed ingredients
Depending on veterinary and managing practices, the availability of feed, and the possible use of antimicrobials or other medicals as prophylactics, there can be high variability in rearing conditions in diverse areas of the world. In the following, two functional feed ingredients with entirely different modes of action are presented that support piglets at different rearing conditions.
7.1 Egg immunoglobulins (IgY) support piglets under poor rearing conditions
Egg immunoglobulins are beneficial if piglets are not raised under the best conditions, meaning lower hygienic standards and higher pathogenic pressure. With egg immunoglobulins coming from hens having been in contact with pathogens relevant to piglets, it is possible to support the young animals. What is the background? Hens are able to transfer maternal antibodies against diseases that they are confronted with to the egg. With this mechanism, they can provide their progeny with a starter kit for the first time after hatching. However, the best thing is that these antibodies are also helpful for mammals.
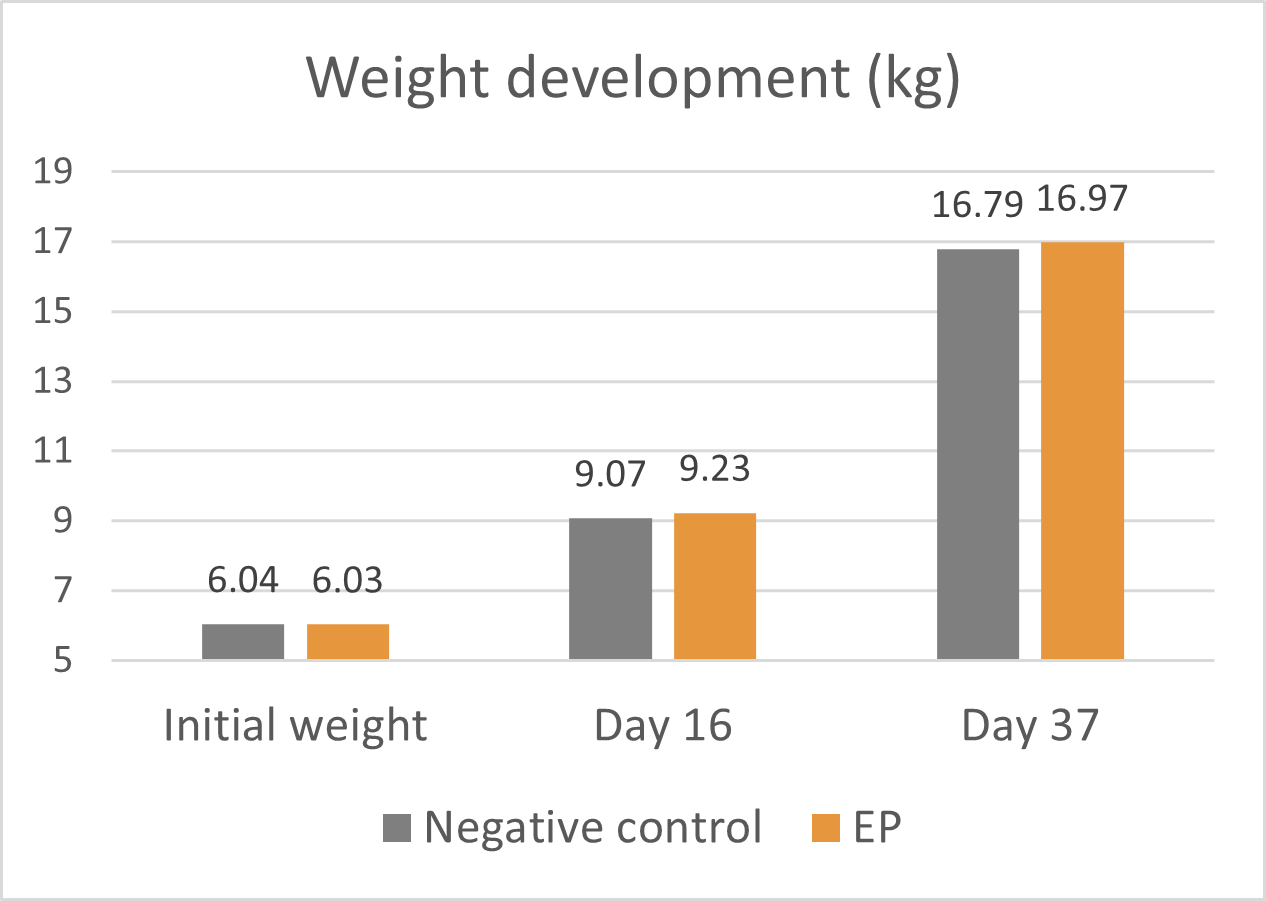
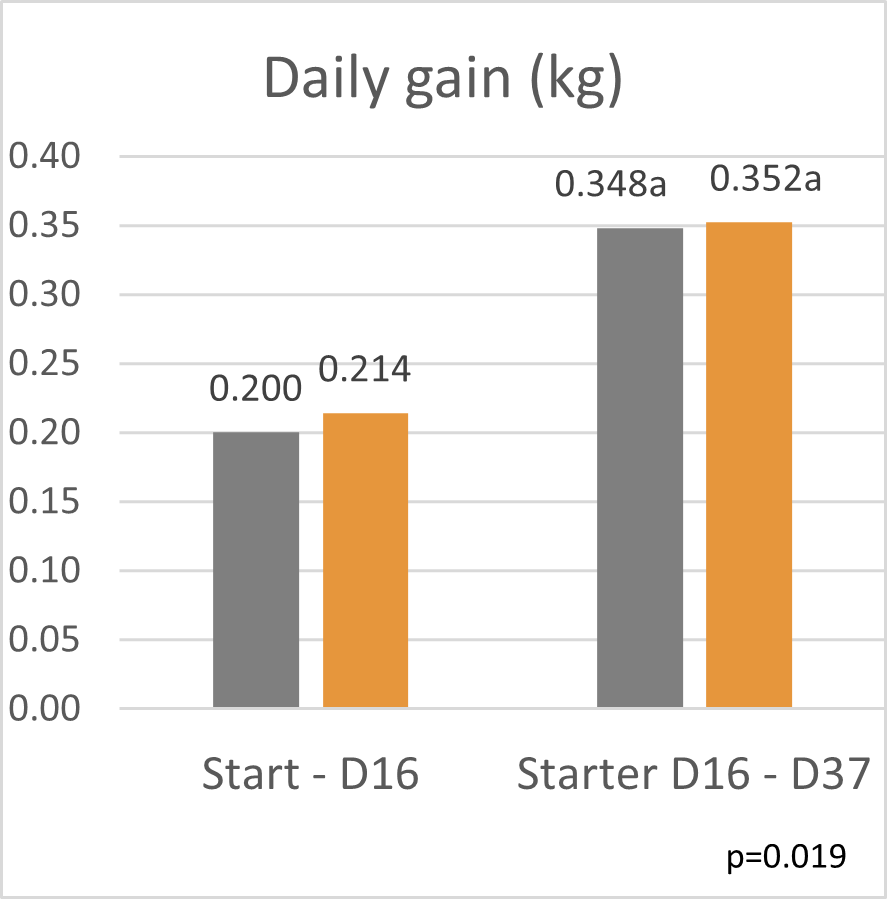
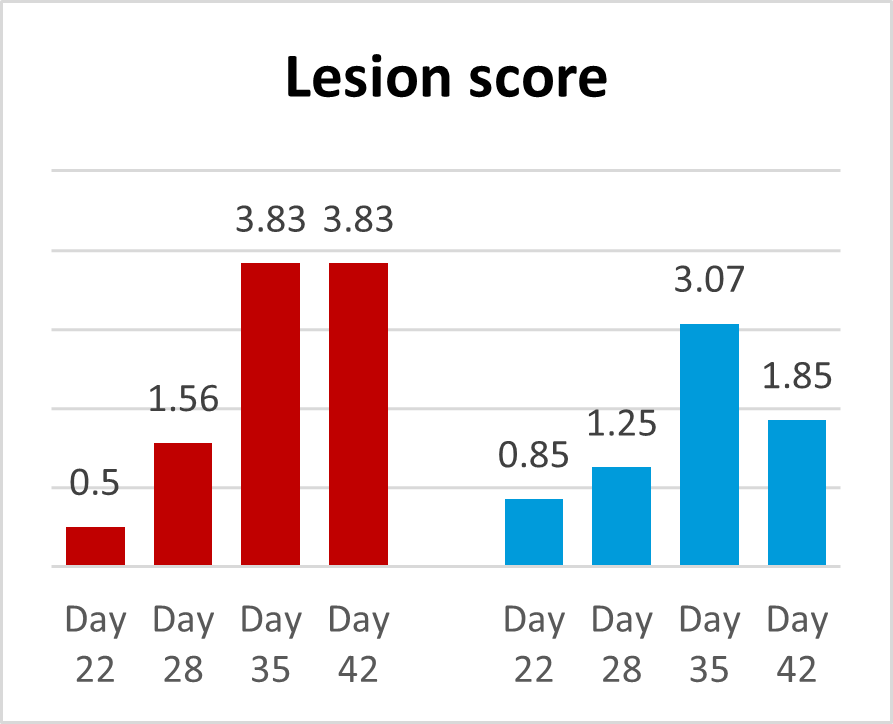
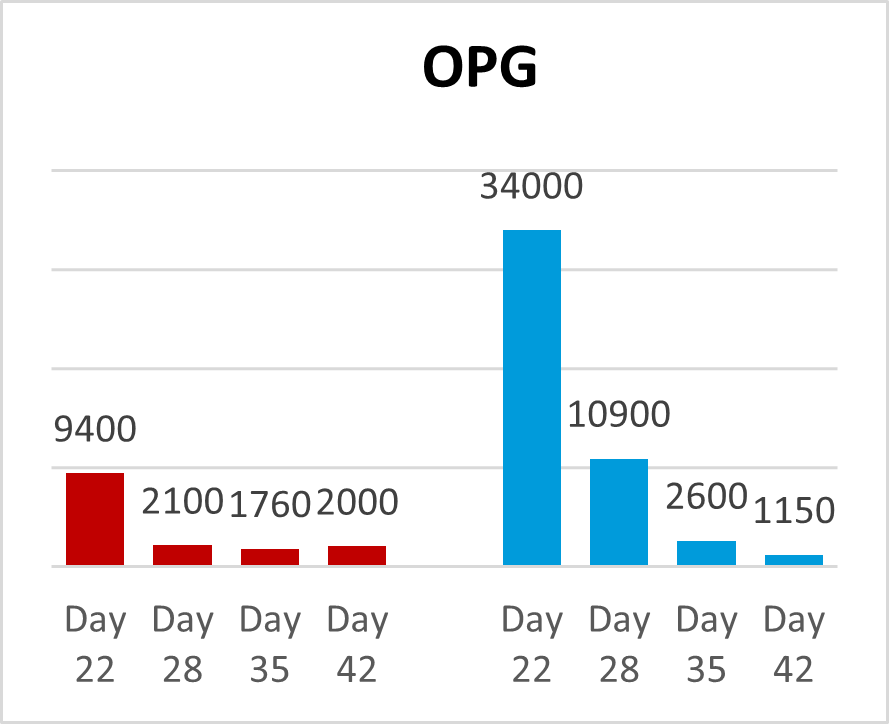
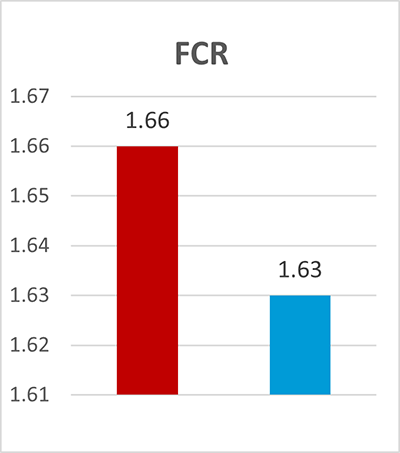
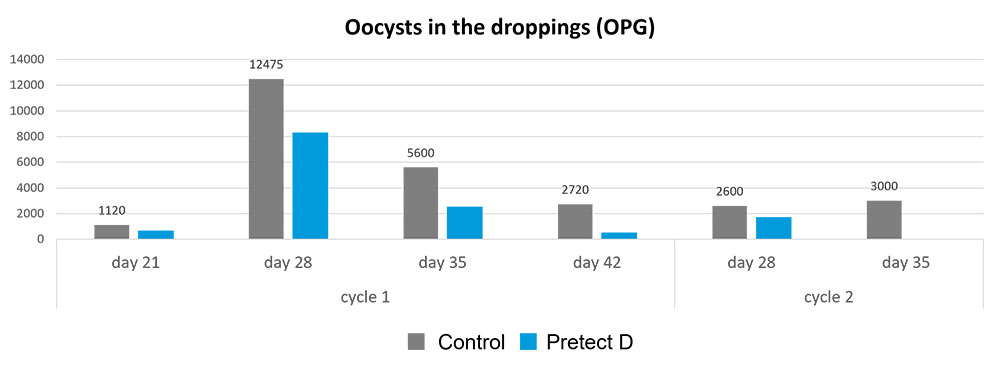
A trial conducted on a commercial farm in Spain shows the weight development of piglets fed an IgY-containing egg powder product (EP) compared to a negative control. The weaned piglets were fed a two-phase feeding (15 days prestarter, 22 days starter). The control (n=51) received no additional functional feed ingredient, whereas the EP group was fed 2 kg of the product/t of feed during the prestarter phase. The animals were weighed individually on days 16 and 37.
The results are shown in Figures 1 and 2.
Explanation of the results: Under poor hygienic conditions, the pathogenic pressure is relatively high, and everything lowering this pressure helps to improve gut health, the utilization of nutrients, and performance. Egg immunoglobulins positively influence the gut microbiome, thus helping reduce diarrhea. By lowering the pathogenic pressure, the organism’s energy can be used for growth and must not be employed for the body’s defense.
7.2 Phytomolecules can even show improvement under optimum conditions
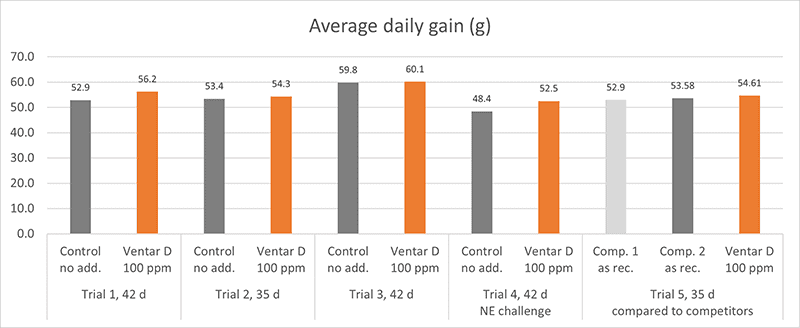
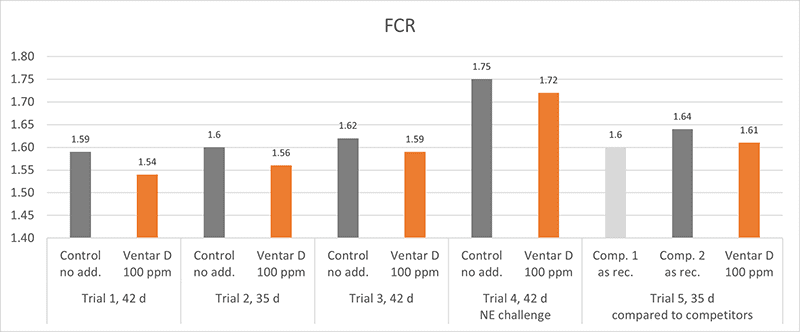
Phytomolecules generally show diverse gut health-promoting effects, from driving the intestinal microbiome in the right direction and strengthening the intestinal barrier to acting as antioxidants or anti-inflammatories or increasing the secretion of digestive juices and, therefore, improving digestion. Which mode of action is relevant if the piglets are raised under already optimal conditions (best hygiene, no prophylactic antibiotics or zinc oxide) and show the highest growth? Is there still room for improvement? Yes, it is. A trial conducted in Germany adduces evidence.
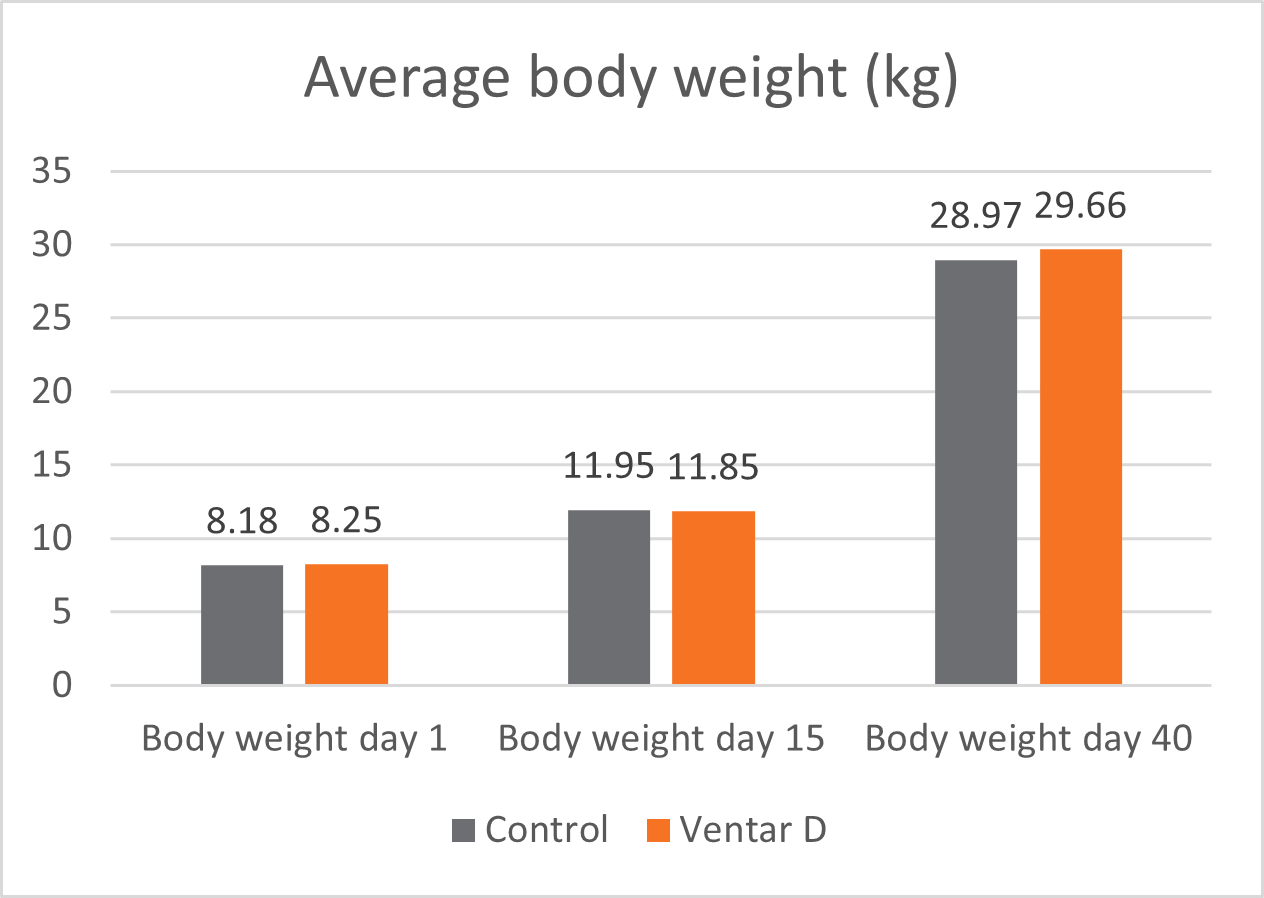
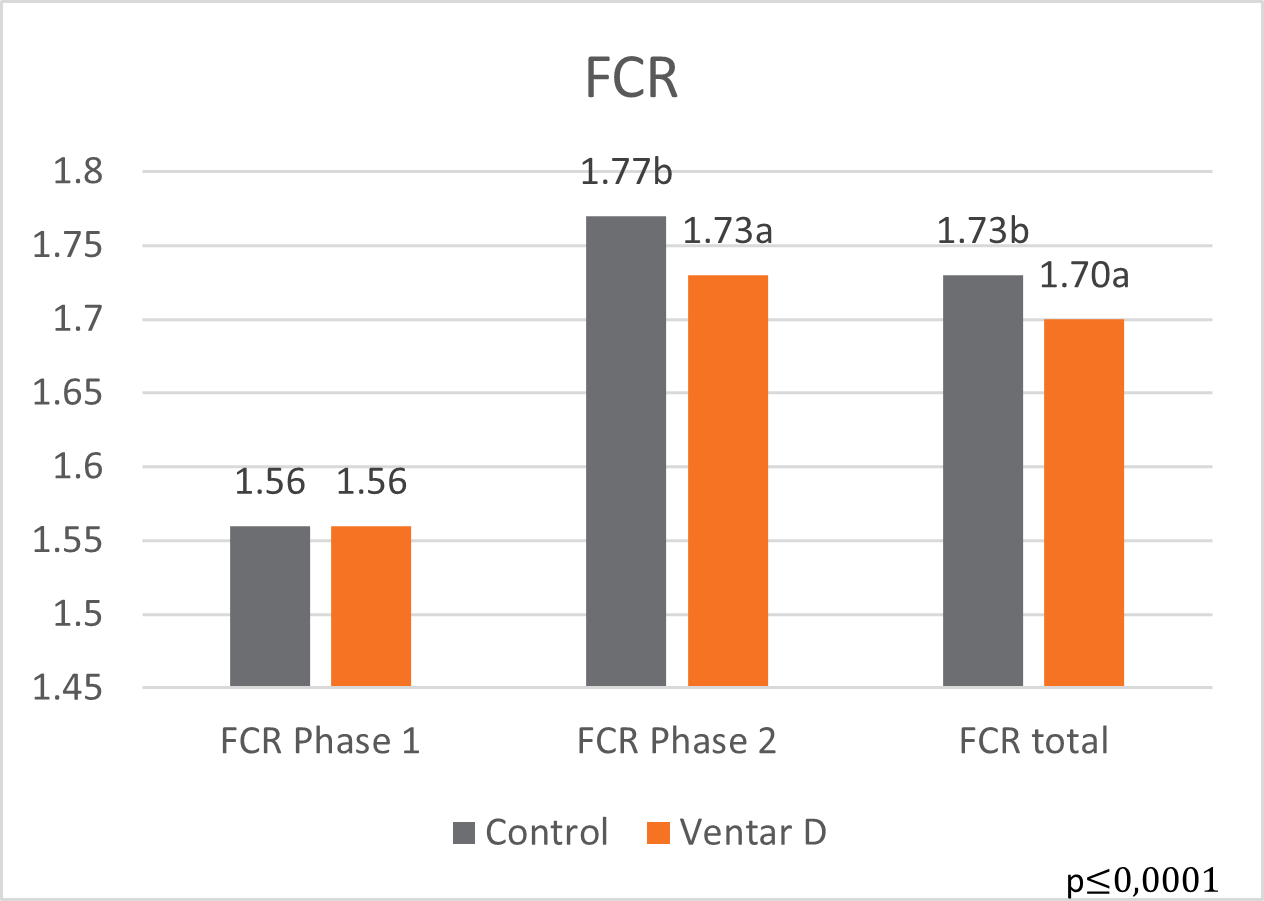
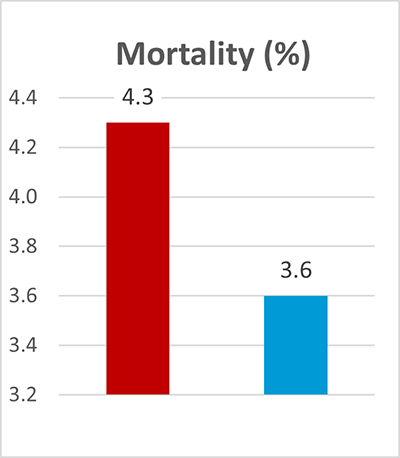
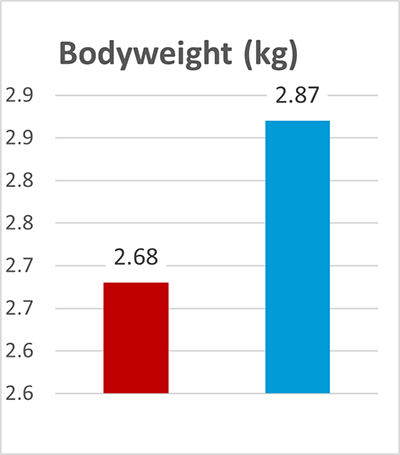
In this trial, 220 piglets weaned on average at 26 days and weighing around 8 kg were housed in 20 pens of 11 castrated males or gilts each. Piglets were blocked by body weight and fed a two-phase feeding program (phase 1 from day 1 to day 13 and phase 2 from day 17 to day 40; pelleted diet). Neither feed or water medication nor therapeutic levels of ZnO were used.
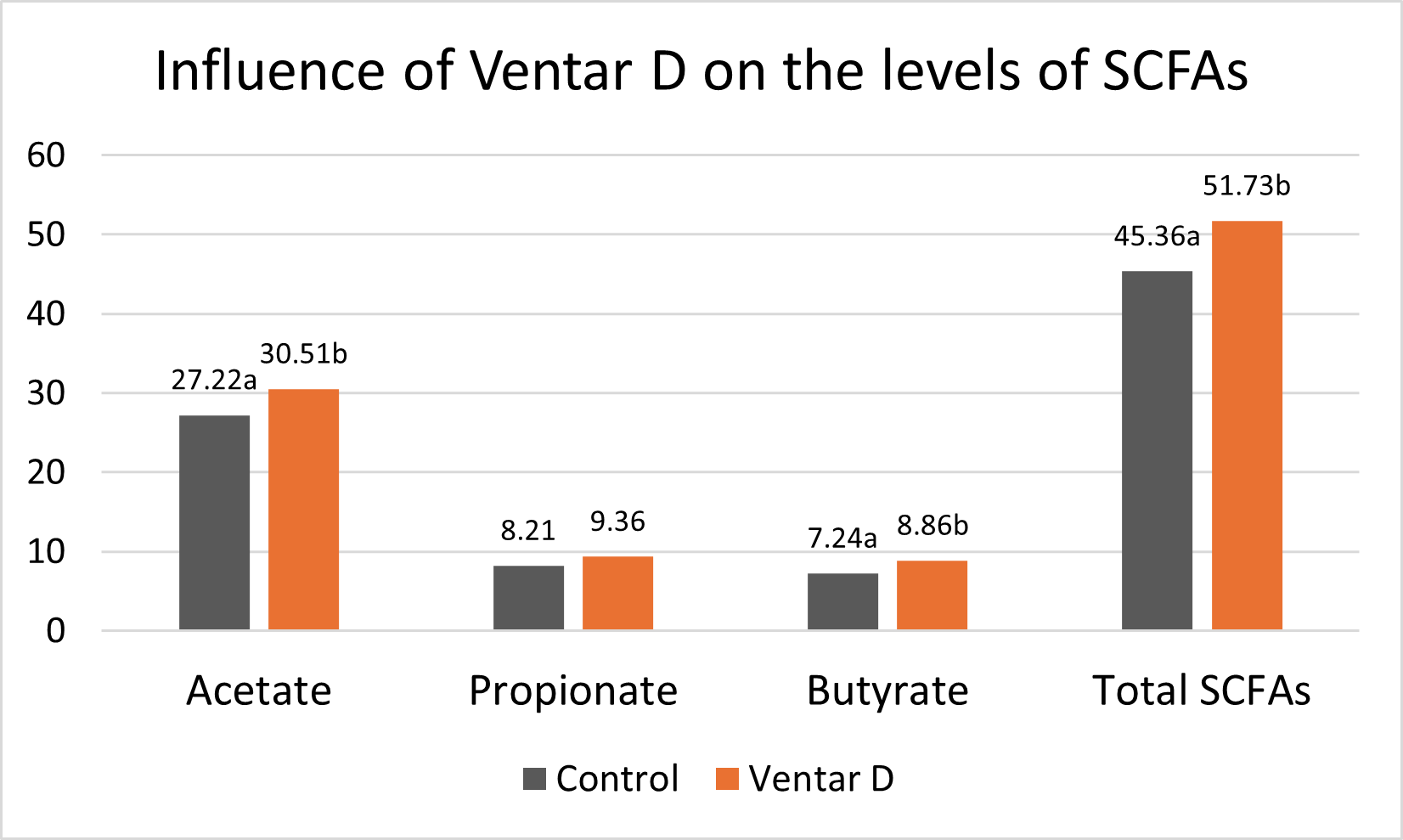
The results of this piglet trial can be seen in Figures 3 and 4.
Explanation of the results: The figures show that the piglets in the control already have an extremely high weight compared to those of a similar age in the previous trial, indicating the best rearing conditions in this trial. But, even here, Ventar D has the capacity to improve performance. Why? High-performing animals stress their body more than low-performing ones. Anabolic processes increase oxidative stress and non-infectious inflammation and burden the immune system. The relevant mode of action of Ventar D is not the gut health-promoting or the antimicrobial one because there is no issue. The relevant modes of action in this case are antioxidant and anti-inflammatory. With these two characteristics, Ventar D still has the capacity to improve the performance of piglets that are already at the top level.
8. Conclusion
For high piglet performance, providing the best possible rearing conditions is essential. However, there are differences concerning these conditions in different areas of the world. Depending on them, different feed strategies can be used. Egg immunoglobulins show the best effects if there is a certain pathogenic pressure. Phytomolecules, however, due to their various modes of action, can be beneficial under different levels in rearing conditions. In a low standard, the antimicrobial and gut health-promoting effect is more relevant; in the case of best conditions, the anti-oxidant and anti-inflammatory effects are decisive.
In summary, it could be said that functional feed ingredients have significant advantages in piglet rearing, but the right choice must be made depending on the prevailing conditions.